Scroll left to review
Neutropenia management strategy for TRODELVY
What to know when developing a neutropenia adverse reaction plan1-3
Patient Education
Educating patients about neutropenia
It’s important to explain the risks of neutropenia to your patients, including when neutropenia may occur and steps that their healthcare team may take to support them. Instruct patients to contact their healthcare provider immediately if they experience any of these signs of infection: fever, chills, cough, shortness of breath, or burning/pain when they urinate.

Be Aware and Prepare
TRODELVY can cause severe, life-threatening, or fatal neutropenia as early as the first cycle of treatment.1
Among patients treated with TRODELVY in the clinical trials:
- Neutropenia occurred in 64% of patients treated with TRODELVY
- 49% of patients experienced Grade 3-4 neutropenia
- 6% of patients experienced febrile neutropenia
- 1.4% of patients experienced neutropenic colitis
The median time to first onset of neutropenia (including febrile neutropenia) in patients receiving TRODELVY was 16 days (range: 1 to 435 days).1,a Neutropenia occurred earlier in patients with reduced UGT1A1 activity.1
aThis includes patients from 4 studies (IMMU-132-01, ASCENT, TROPiCS-02, and a phase 2 trial in another tumor type).1
Median time to onset and duration of any grade neutropenia
bEvents of "neutropenia" included the preferred terms "neutropenia" and "neutrophil count decreased" in both studies, as well as "febrile neutropenia" in TROPiCS-02.3,4
Primary prophylaxis with G-CSF is recommended in the TRODELVY USPI starting in the first cycle of treatment for all patients at an increased risk of febrile neutropenia, including older patients, patients with previous neutropenia, poor performance status, organ dysfunction, or multiple comorbidities. Withhold TRODELVY for neutropenic fever.1
Don’t delay a prior authorization request for your patient, as it may be required
for G-CSF.
MANAGEMENT STRATEGIES
Strategies to help proactively monitor and manage neutropenia
When a patient starts receiving TRODELVY, it is key to stay alert to any adverse reactions they may experience. This can help you tailor a plan to each patient.

Monitor
Monitor absolute neutrophil count (ANC) periodically during treatment.1 Inform the patient about the lab work they may expect.
Encourage patients to tell their healthcare team right away if they develop any of the following signs of infection during treatment with TRODELVY1:
- Fever
- Chills
- Cough
- Shortness of breath
- Burning pain when urinating
NCI CTCAE Version 5.0 neutropenia grade scale5 |
|
|---|---|
|
Grade 1 |
ANC <LLN to 1500/mm3 |
|
Grade 2 |
ANC <1500 to 1000/mm3 |
|
Grade 3 |
ANC <1000 to 500/mm3 |
|
Grade 4 |
ANC <500/mm3 |
ANC=absolute neutrophil count; LLN=lower limit of normal.
NCI CTCAE Version 5.0 febrile neutropenia grade scale5 |
|
|---|---|
Grade 1 |
- |
Grade 2 |
- |
Grade 3 |
ANC <1000/mm3 with a single temperature of >38.3 °C (101 °F) or a sustained temperature of ≥38 °C (100.4 °F) for more than 1 hour |
Grade 4 |
Life-threatening consequences; urgent intervention indicated |
Grade 5 |
Death |
ANC=absolute neutrophil count.

Manage
To manage Grade 3-4 neutropenia (ANC <1000/mm3) or febrile neutropenia1:

Withhold TRODELVY until ANC reaches the following levels
Day 1 dose: ANC ≥1500/mm3 or
Day 8 dose: ANC ≥1000/mm3

Administer G-CSF during treatment as clinically indicated

Reduce 1 dose level for each occurrence of febrile neutropenia or prolonged Grade 3-4 neutropenia, or discontinue according to the dosage reduction levels information below
Dosage reduction levels1
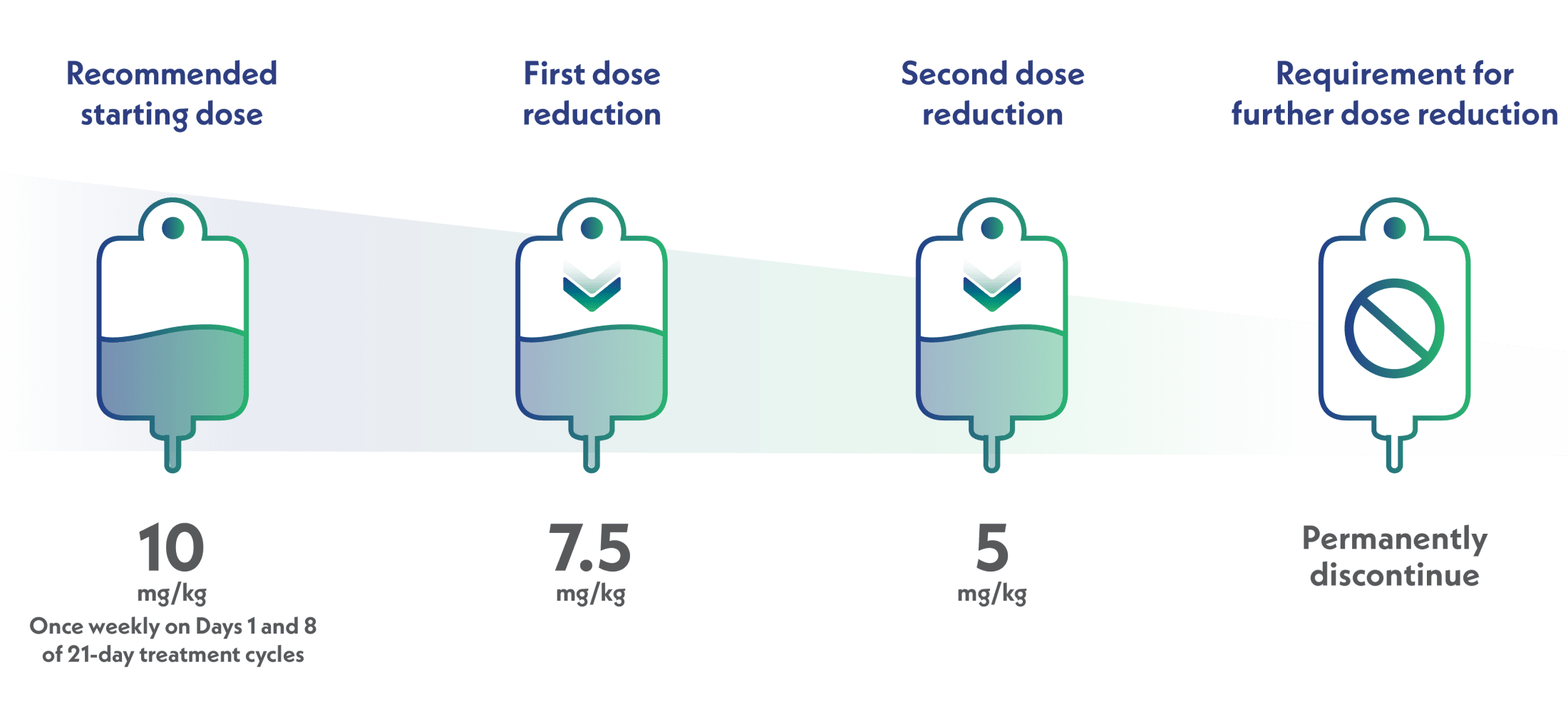
Scroll left to review
Recommended
starting dose
First dose
reduction
Second dose
reduction
Requirement for
further dose reduction
Do not reescalate the TRODELVY dose after a dose reduction for adverse reactions has been made.1
If patients experience neutropenia, be ready with G-CSF support to help
patients continue therapy if clinically indicated
Explore management strategies for neutropenia and certain other adverse reactions of TRODELVY in a personalized interactive experience
VIDEO OVERVIEW
From planning to practice: Adverse reaction management strategies
With Dr. Sydney Schultz, PharmD, RPh, BCOP, and Dr. Kim Nguyen, PharmD, APh, BCOP
<B-ROLL VIDEO>
<VO: MUSIC>
Green screen with DR. SYDNEY SCHULTZ and DR. KIM NGUYEN sitting down on-set, getting ready for their discussion. Film crew and equipment (green screen, cameras, microphones, lights, etc) can be seen in background. Wide camera angle captures studio briefly before transitioning to green screen-edited video content.
<END OF B-ROLL VIDEO>
<TEXT ON-SCREEN>
[IMAGE: TRODELVY LOGO]
FROM PLANNING TO PRACTICE:
ADVERSE REACTION MANAGEMENT STRATEGIES
<END OF VO: MUSIC>
<TEXT ON-SCREEN>
<VO>
INDICATIONS
TRODELVY® (sacituzumab govitecan-hziy) is a Trop-2-directed antibody and topoisomerase inhibitor conjugate indicated for the treatment of adult patients with:
- Unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
- Unresectable locally advanced or metastatic hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative (IHC 0, IHC 1+ or IHC 2+/ISH–) breast cancer who have received endocrine-based therapy and at least two additional systemic therapies in the metastatic setting.
<END OF TEXT ON-SCREEN>
<TEXT ON-SCREEN>
<VO>
IMPORTANT SAFETY INFORMATION
- TRODELVY can cause severe, life-threatening, or fatal neutropenia. Withhold TRODELVY for absolute neutrophil count below 1500/mm3 or neutropenic fever. Monitor blood cell counts periodically during treatment. Primary prophylaxis with G-CSF is recommended for all patients at increased risk of febrile neutropenia. Initiate anti-infective treatment in patients with febrile neutropenia without delay.
- TRODELVY can cause severe diarrhea. Monitor patients with diarrhea and give fluid and electrolytes as needed. At the onset of diarrhea, evaluate for infectious causes and, if negative, promptly initiate loperamide. If severe diarrhea occurs, withhold TRODELVY until resolved to ≤Grade 1 and reduce subsequent doses.
<TEXT ON-SCREEN>
Please continue watching for Important Safety Information at the end of this video, and please click the link provided for full Prescribing Information, including BOXED WARNING.
<END OF TEXT ON-SCREEN>
<VO>
TRODELVY has a boxed warning.
<TEXT ON-SCREEN>
Continue watching for Important Safety Information at the end of this video, and please see Full Prescribing Information, including BOXED WARNING.
<END OF TEXT ON-SCREEN>
<END OF VO: ISI/BOXED WARNING>
<CAMERA STARTS WITH BOTH EXPERTS. CUT TO DR. SCHULTZ, TIGHT SHOT>
<TEXT ON-SCREEN>
Dr. Sydney Schultz, PharmD, RPh, BCOP
Clinical Oncology Pharmacist
<VO: Dr. Schultz>
Welcome! I’m Dr. Sydney Schultz, a clinical oncology pharmacist at an academic medical center in Minnesota.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Schultz>
<CAMERA CUTS TO DR. NGUYEN, TIGHT SHOT>
<TEXT ON-SCREEN>
Dr. Kim Nguyen, PharmD, APh, BCOP
Clinical Oncology Pharmacist
<VO: Dr. Nguyen>
And I’m Dr. Kim Nguyen. I’m also a clinical oncology pharmacist at an academic medical center in California.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Nguyen>
<CAMERA CUTS TO BOTH EXPERTS, WIDE SHOT>
<VO: Dr. Schultz>
In this video, we’ll be sharing insights about how to prepare for and potentially manage select adverse reactions with TRODELVY. We’ll also be following 2 patient cases as they receive TRODELVY and discussing the strategies their care teams used to manage certain adverse reactions. Please pause this video at any point if you need more time to read what’s on screen.
<END OF VO: Dr. Schultz>
<TEXT ON-SCREEN>
Please pause this video at any point if you need more time to read what’s on screen.
<END OF TEXT ON SCREEN>
<VO: Dr. Nguyen>
Before patients receive their first cycle of TRODELVY, there are a few conversations I like to have, including informing patients about adverse reactions that can occur with TRODELVY.
<END OF VO: Dr. Nguyen>
<TEXT ON-SCREEN>
BE AWARE
<END OF TEXT ON SCREEN>
<VO: Dr. Schultz>
Let’s take a moment to review some information patients should be aware of.
TRODELVY can cause serious adverse reactions, including severe, life-threatening, or fatal neutropenia; severe diarrhea; serious hypersensitivity and infusion-related reactions, including life-threatening anaphylactic reactions; and severe nausea and vomiting.
<TEXT ANIMATES IN>
TRODELVY can cause:
- Severe, life-threatening, or fatal neutropenia
- Severe diarrhea
- Serious hypersensitivity and infusion-related reactions, including life-threatening anaphylactic reactions
- Severe nausea and vomiting
<END OF TEXT ON SCREEN>
<VO: Dr. Schultz>
Among patients treated with TRODELVY in the clinical trials, here are the most common adverse reactions—including laboratory abnormalities—reported in 25% or more of patients.
<TEXT ON-SCREEN>
Among patients treated with TRODELVY in the clinical trials, the most common adverse reactions (including laboratory abnormalities) reported in ≥25% of patients were:
- Decreased leukocyte count (84%)
- Decreased neutrophil count (75%)
- Decreased hemoglobin (69%)
- Diarrhea (64%)
- Nausea (64%)
- Decreased lymphocyte count (63%)
- Fatigue (51%)
- Alopecia (45%)
- Constipation (37%)
- Increased glucose (37%)
- Decreased albumin (35%)
- Vomiting (35%)
- Decreased appetite (30%)
- Decreased creatinine clearance (28%)
- Increased alkaline phosphatase (28%)
- Decreased magnesium (27%)
- Decreased potassium (26%)
- Decreased sodium (26%)
<VO: Dr. Schultz>
These should be shared with your patients before their first infusion.
We'll hold for a moment to give you more time to read what's on screen.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Schultz>
<TEXT ANIMATES IN>
<TEXT ON-SCREEN>
PREPARE
<VO: Dr. Nguyen>
Once I’ve had this discussion with my patients, we can start thinking about how to help them prepare for certain adverse reactions.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Nguyen>
<CAMERA CUTS TO BOTH EXPERTS, WIDE SHOT>
<VO: Dr. Schultz>
What strategies do you use?
<END OF VO: Dr. Schultz>
<VO: Dr. Nguyen>
I start by talking to patients about what adverse reactions they may have experienced with previous treatments.
I ask if they have a history of neutropenia, neutropenic fever, excessive cholinergic response, any infusion-related reactions, or nausea and vomiting.
<END OF VO: Dr. Nguyen>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Understanding adverse reactions with past treatments
Any history of:
- Neutropenia or neutropenic fever?
- Excessive cholinergic response?
- Infusion-related reactions?
- Nausea or vomiting?
<END OF TEXT ON SCREEN>
<CAMERA CUTS TO BOTH EXPERTS, WIDE SHOT>
<VO: Dr. Schultz>
I ask those questions, too. Collecting baseline information can help you track any changes during treatment.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Understanding a patient’s baseline
- Consider documenting baseline bowel movements
- Consider documenting baseline performance status
<VO: Dr. Schultz>
For example, with diarrhea, you may want to document baseline bowel movements, and for nausea, I like to understand any known triggers so I can work with the patient to develop a plan to potentially avoid or mitigate them.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Schultz>
<CAMERA CUTS TO DR. NGUYEN>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Discuss management strategies for certain adverse reactions
<VO: Dr. Nguyen>
Next, I discuss strategies that can help prevent or manage certain adverse reactions with TRODELVY.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Nguyen>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Primary prophylaxis with G-CSF is recommended starting in the first cycle for all patients at an increased risk of febrile neutropenia
G-CSF=granulocyte colony-stimulating factor.
<END OF TEXT ON SCREEN>
<VO: Dr. Schultz>
Prophylaxis for neutropenia is an example of this. The TRODELVY Prescribing Information recommends primary prophylaxis with G-CSF as early as the first cycle for all patients at increased risk of febrile neutropenia. I let my patients know the recommendation and have a conversation with them to assess whether they’re at an increased risk for febrile neutropenia.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
- older patients
- previous neutropenia
- poor performance status
- organ dysfunction
- multiple comorbidities
<VO: Dr. Schultz>
Patients at increased risk include older patients, patients with previous neutropenia, poor performance status, organ dysfunction, or multiple comorbidities.
<END OF TEXT ON SCREEN>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Plan for G-CSF support
Treat neutropenia with G-CSF and administer prophylaxis in subsequent cycles as clinically indicated.
<VO: Dr. Schultz>
If patients did not receive primary prophylaxis with G-CSF and experience neutropenia, dose modifications may be required. Be ready with G-CSF support to help patients stay on therapy, if clinically indicated.
<END OF VO: Dr. Schultz>
<CAMERA CUTS TO DR. NGUYEN>
<VO: Dr. Nguyen>
When it comes to G-CSF, we also must think about if short-acting or long-acting G-CSF is most appropriate for the patient.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Short-acting
Long-acting
<VO: Dr. Nguyen>
Short-acting G-CSF products like filgrastim are administered daily while long-acting products like pegfilgrastim stay in the body longer and can be given less frequently.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Short-acting
- Administered daily
Long-acting
- Stays in the body longer
- Can be given less frequently
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Nguyen>
<CAMERA CUTS TO DR. SCHULTZ>
<VO: Dr. Schultz>
When choosing between G-CSF formulations, I like to consider the cost, convenience, and adherence.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
- Cost
- Convenience
- Adherence
<VO: Dr. Schultz>
On top of these, you should check which options are covered by your patient’s insurance plan. I work with my patients so I can be confident that we are selecting the option that is the best fit for them.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Schultz>
<CAMERA CUTS TO BOTH EXPERTS, WIDE SHOT>
<VO: Dr. Nguyen>
Getting on the same page about which medications patients are prescribed and how to take them is an important step in helping patients prepare for TRODELVY.
<END OF VO: Dr. Nguyen>
<VO: Dr. Schultz>
Aside from G-CSF, there are other preventative measures that are recommended prior to administering TRODELVY.
<END OF VO: Dr. Schultz>
<VO: Dr. Nguyen>
To help prevent infusion-related reactions, it’s recommended that patients are given antipyretics and H1 and H2 blockers prior to infusion.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Prevention of infusion-related reactions
- Antipyretics
- H1 and H2 blockers
- Corticosteroids may be used for patients who had a prior infusion reaction
5-HT3=5-hydroxytryptamine type 3 receptor; CINV=chemotherapy-induced nausea and vomiting; H1=histamine receptor 1; H2=histamine receptor 2; NK1=neurokinin-1.
<VO: Dr. Nguyen>
Corticosteroids may be used for patients who had a prior infusion reaction.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Prevention of infusion-related reactions
- Antipyretics
- H1 and H2 blockers
- Corticosteroids may be used for patients who had a prior infusion reaction
Prevention of chemotherapy-induced nausea and vomiting
- Can include premedication with a 2- or 3-drug combination (eg, dexamethasone with either a 5-HT3 receptor antagonist or an NK1 receptor antagonist, as well as other drugs as indicated)
5-HT3=5-hydroxytryptamine type 3 receptor; H1=histamine receptor 1; H2=histamine receptor 2; NK1=neurokinin-1.
<VO: Dr. Nguyen>
For prevention of chemotherapy-induced nausea and vomiting, premedicate with a 2- or 3-drug regimen.
For example, this can be dexamethasone with either a 5-HT3 receptor antagonist or an NK1 receptor antagonist, as well as other drugs as indicated.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Nguyen>
<CAMERA CUTS TO BOTH EXPERTS, WIDE SHOT>
<VO: Dr. Schultz>
When deciding which premedications to prescribe, it’s important to have a conversation with patients, including how any previous premedications may have worked for them. Because every patient is unique, we need to find the approach that works best for them.
<END OF VO: Dr. Schultz>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
DOSING
<END OF TEXT ON SCREEN>
<VO: Dr. Nguyen>
Let’s take a look at the dosing and infusion schedule.
<CAMERA CUTS TO DR. NGUYEN>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Start TRODELVY at 10 mg/kg
<VO: Dr. Nguyen>
The starting dosage of TRODELVY is 10 milligrams per kilogram. Do not administer TRODELVY at doses greater than 10 milligrams per kilogram.
<TEXT ON-SCREEN>
Start TRODELVY at 10 mg/kg
Administer TRODELVY as an intravenous infusion only. Do not administer TRODELVY at doses greater than 10 mg/kg. Do not administer as an intravenous push or bolus.
Do NOT substitute TRODELVY for or use with other drugs containing irinotecan or its active metabolite SN-38.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Nguyen>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
[graphic]
10 mg/kg
10 mg/kg
Continue treatment until disease progression or unacceptable toxicity.
<VO: Dr. Schultz>
TRODELVY is given intravenously on Days 1 and 8 of a 21-day continuous treatment cycle. Treatment is continued until disease progression or unacceptable toxicity.
<END TEXT ON SCREEN>
<END OF VO: Dr. Schultz>
<VO: Dr. Nguyen>
The first infusion of TRODELVY is administered over 3 hours, and if well-tolerated, subsequent infusions are administered over 1 to 2 hours.
<TEXT ANIMATES ON SCREEN>
First infusion
Administer over 3 hrs
IF WELL TOLERATED
Subsequent infusions
Administer over 1-2 hrs
Observe patients during the infusion and for at least 30 minutes following the infusion for signs and symptoms of infusion-related reactions.
<END TEXT ON SCREEN>
<END OF VO: Dr. Nguyen>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Closely monitor patients for hypersensitivity and infusion-related reactions during each infusion and for at least 30 minutes after the infusion is complete.
<VO: Dr. Schultz>
Closely monitor patients for hypersensitivity and infusion-related reactions during each infusion of TRODELVY and for at least 30 minutes after each infusion is complete.
<END TEXT ON SCREEN>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
30 minutes
<END TEXT ON SCREEN>
<END OF VO: Dr. Schultz>
<CAMERA CUTS TO BOTH EXPERTS, WIDE SHOT>
<VO: Dr. Nguyen>
Now that we’ve discussed the recommended dosage, let’s take a moment to go over any dose modifications that may be necessary due to adverse reactions.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
DOSE MODIFICATIONS FOR ADVERSE REACTIONS
Management of adverse reactions may require temporary interruption, dose reduction, or treatment discontinuation of TRODELVY.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Nguyen>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
For Grade 3-4 neutropenia (ANC <1000/mm3) or febrile neutropenia, withhold TRODELVY until ANC is ≥1500/mm3 for a patient’s Day 1 dose or ≥1000/mm3 for a patient’s Day 8 Dose
<VO: Dr. Schultz>
For Grade 3 to 4 neutropenia, or febrile neutropenia, TRODELVY should be withheld until ANC is at least 1500 per cubic millimeter for a patient’s Day 1 dose or at least 1000 per cubic millimeter for a patient’s Day 8 dose, and G-CSF should be administered during treatment as clinically indicated.
<TEXT ON-SCREEN>
For Grade 3-4 neutropenia (ANC <1000/mm3) or febrile neutropenia, withhold TRODELVY until ANC is ≥1500/mm3 for a patient’s Day 1 dose or ≥1000/mm3 for a patient’s Day 8 Dose
G-CSF should be administered during treatment as clinically indicated.
<END OF TEXT ON SCREEN>
<TEXT ON-SCREEN>
Reduce one dose level with each occurrence of febrile neutropenia or prolonged Grade 3-4 neutropenia:
Recommended starting dose
10 mg/kg
- Once weekly on Days 1 and 8 of 21-day treatment cycles
First dose reduction
7.5 mg/kg
Second dose reduction
5 mg/kg
Requirement for further dose reduction
Permanently discontinue
Do not re-escalate the TRODELVY dose after a dose reduction for adverse reactions has been made ANC=absolute neutrophil count.
<VO: Dr. Schultz>
For each occurrence of febrile neutropenia or prolonged Grade 3 to 4 neutropenia, the dose should be reduced one level or discontinued according to the following dose reduction guidance.
From the recommended starting dose of 10 milligrams per kilogram once weekly on Days 1 and 8 of a 21-day treatment cycle, reduce to 7.5 milligrams per kilogram for the first occurrence; reduce to 5 milligrams per kilogram for the second occurrence; and permanently discontinue for any further dose reduction requirements.
Do not re-escalate the TRODELVY dose after a dose reduction for adverse reactions has been made.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Schultz>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
For Grade 3-4 nausea, vomiting, or diarrhea that is not controlled with antiemetics or anti-diarrheal agents, withhold TRODELVY until resolved to ≤Grade 1
<VO: Dr. Nguyen>
For Grade 3 to 4 nausea, vomiting, or diarrhea that is not controlled with antiemetics or anti-diarrheal agents, TRODELVY should be withheld at the time of scheduled treatment and resumed when resolved to Grade 1 or lower.
<TEXT ON-SCREEN>
For Grade 3 to 4 nausea, vomiting, or diarrhea that is not controlled with antiemetics or anti-diarrheal agents, withhold TRODELVY until resolved to ≤Grade 1
Reduce one dose level with each occurrence:
Recommended starting dose
10 mg/kg
- Once weekly on Days 1 and 8 of 21-day treatment cycles
First dose reduction
7.5 mg/kg
Second dose reduction
5 mg/kg
Requirement for further dose reduction
Permanently discontinue
Do not re-escalate the TRODELVY dose after a dose reduction for adverse reactions has been made
<VO: Dr. Nguyen>
Reduce one dose level with each occurrence, following the same sequence of dose reduction or discontinuation.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Nguyen>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Infusion-related reactions
Grades 1-3: Slow or interrupt the infusion rate of TRODELVY
Grade 4: Discontinue TRODELVY
<VO: Dr. Schultz>
For Grade 1 to 3 infusion-related reactions, slow the infusion rate or interrupt the infusion of TRODELVY.
For Grade 4 infusion-related reactions, discontinue TRODELVY.
Remember to always have medication and emergency equipment immediately available to treat infusion-related reactions including anaphylaxis.
<END OF TEXT ON SCREEN>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
For other Grade 3-4 toxicities of any duration despite optimal medical management, withhold TRODELVY until resolved to ≤Grade 1
<VO: Dr. Schultz>
For other Grade 3 or 4 toxicities of any duration, despite optimal medical management, TRODELVY should be withheld at the time of scheduled treatment and resumed when resolved to Grade 1 or lower.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
For other Grade 3-4 toxicities of any duration despite optimal medical management, withhold TRODELVY until resolved to ≤Grade 1
Reduce one dose level with each occurrence:
Recommended starting dose
10 mg/kg
Once weekly on Days 1 and 8 of 21-day treatment cycles
First dose reduction
7.5 mg/kg
Second dose reduction
5 mg/kg
Requirement for further dose reduction
Permanently discontinue
Do not re-escalate the TRODELVY dose after a dose reduction for adverse reactions has been made
<VO: Dr. Schultz>
Reduce one dose level with each occurrence following the sequence of dose reduction or discontinuation, as seen on screen.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Schultz>
<CAMERA CUTS TO BOTH EXPERTS, WIDE SHOT>
<VO: Dr. Nguyen>
I know dose modification may make patients feel nervous, skeptical, or frustrated. Many worry that a lower dose might compromise effectiveness, while others may perceive it as a setback.
How do you approach conversations with patients about dose reductions?
<END OF VO: Dr. Nguyen>
<VO: Dr. Schultz>
The reality is that the managements of adverse reactions may require temporary interruption, dose reduction, or treatment discontinuation of TRODELVY, and having these conversations early on can help patients prepare for changes that may lie ahead.
<TEXT ON SCREEN>
Management of adverse reactions may require temporary interruption, dose reduction, or treatment discontinuation of TRODELVY.
<VO: Dr. Schultz>
I find it helpful to explain to patients that a dose modification is not a step backward. It’s just a part of finding the best approach to treatment for each person.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Schultz>
<VO: Dr. Nguyen>
I tell my patients that it’s NOT a reduction in care. It’s not a sign that the treatment isn’t working or that they have done anything wrong. It’s a personalized refinement of the treatment plan to help meet their treatment goals.
Let’s have a look at how the information we’ve discussed might apply to patients using 2 patient cases, starting with Dana.
<END OF VO: Dr. Nguyen>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
PATIENT CASE: DANA
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Nguyen>
<CAMERA CUTS TO DR. NGUYEN>
<GRAPHICS ANIMATE ON SCREEN>
<VO: Dr. Nguyen>
Dana is a 52-year-old African American woman who was diagnosed with HER2-low IHC 1+ metastatic TNBC.
<TEXT ON-SCREEN>
Actor portrayal
Dana
Age: 52
Race/ethnicity: African American
Has HER2-low IHC 1+ mTNBC that has metastasized to her liver, bones, and lymph nodes
Metastatic treatment history:
- Chemotherapy for 6 months
Hypothetical patient case for illustrative purposes only. Information in this program does not constitute the provision of medical advice and should not substitute for clinical decision-making.
HER2=human epidermal growth factor receptor 2; IHC=immunohistochemistry; mTNBC=metastatic triple-negative breast cancer.
<VO: Dr. Nguyen>
Dana had undergone a chemotherapy in the metastatic setting for 6 months before her disease progressed. After discussions with her care team, Dana was prescribed TRODELVY for her second-line treatment.
<END OF TEXT ON SCREEN>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Actor portrayal
Dana
Adverse reactions experienced on prior therapies:
- Neutropenia
- Thrombocytopenia
- Fatigue
Other considerations
- Open wounds from a recent Mohs procedure due to suspicious skin lesion
<VO: Dr. Nguyen>
Dana’s healthcare team speaks with her about her medical history and learns that she experienced neutropenia, thrombocytopenia, and fatigue on prior treatments.
Dana had multiple risk factors for febrile neutropenia, including an open wound as a result of a Mohs procedure, prior neutropenia, and prior chemotherapy. Based on this assessment, Dana is given long-acting G-CSF as primary prophylaxis.
<END OF TEXT ON SCREEN>
<VO: Dr. Nguyen>
Prior to her first dose with TRODELVY, Dana receives premedications for the prevention of infusion-related reactions and nausea and vomiting.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Patient counseling tip:
Instruct patients to contact their healthcare provider immediately the first time during treatment they experience diarrhea; black or bloody stools; symptoms of dehydration, such as lightheadedness, dizziness, or faintness; inability to take fluids by mouth due to nausea or vomiting; or inability to get diarrhea under control within 24 hours.
<VO: Dr. Nguyen>
Dana is advised to contact her physician immediately the first time during treatment she experiences diarrhea; black or bloody stools; symptoms of dehydration, such as lightheadedness, dizziness, or faintness; inability to take fluids by mouth due to nausea or vomiting; or inability to get diarrhea under control within 24 hours.
<END OF TEXT ON SCREEN>
<GRAPHIC ANIMATES ON SCREEN>
<TEXT ON SCREEN>
Dana’s first cycle of TRODELVY
DOSE 1
DOSE 2
<VO: Dr. Nguyen>
On day 14 of her first treatment cycle, Dana experiences Grade 2 diarrhea. She is having 4 to 6 bowel movements per day. Despite previous advice on when to contact her doctor, Dana decides to wait a day before letting her medical team know.
The next day, her diarrhea gets worse. She is experiencing 10 bowel movements a day, so she calls into the clinic. It's important to note that at the onset of diarrhea, the first step is to determine if there are infectious causes.
<END OF TEXT ON SCREEN>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Have you been exposed to infections recently, such as any recent travel or contact with someone sick?
No
<VO: Dr. Nguyen>
Dana’s care team asks her if she has had any exposures to infections recently.
<END OF TEXT ON SCREEN>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Have you experienced any additional symptoms, such as abdominal cramping or more saliva than usual?
No
<VO: Dr. Nguyen>
I would also want to know about any additional symptoms such as abdominal cramping or increased salivation to determine if she is having a cholinergic response.
<TEXT ON-SCREEN>
Patients who exhibit an excessive cholinergic response to treatment with TRODELVY (eg, abdominal cramping, diarrhea, salivation, etc) can receive appropriate premedication (eg, atropine) for subsequent treatments. Additional supportive measures (e.g., fluid and electrolyte substitution) may also be employed as clinically indicated.
<VO: Dr. Nguyen>
Patients who exhibit an excessive cholinergic response to treatment with TRODELVY (such as abdominal cramping, diarrhea, salivation, or other responses) can receive appropriate premedication (such as atropine) for subsequent treatments.
To understand the severity of the diarrhea, I will also ask what her baseline number of bowel movements is and how many she is currently experiencing.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
How many stools are normal for you and how many did you have yesterday?
Normal for me is 0-1 per day, and yesterday I had diarrhea 10 times
<END OF TEXT ON SCREEN>
<VO: Dr. Nguyen>
Dana states she hasn’t traveled, had any known exposures, or experienced any new symptoms.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
- No travel
- No other exposures to infectious agents
- No additional symptoms
- Normally has 0-1 bowel movements a day, had 10 yesterday
<VO: Dr. Nguyen>
Diagnostic assessments may also be required such as repeat CBCs, body temperature, or other tests based on history and physical exam.
<END OF TEXT ON SCREEN>
<TEXT ON-SCREEN>
Possible diagnostic assessments
<END OF TEXT ON SCREEN>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Test results
Repeat complete blood count (CBC) with differential when clinically indicated
CBC within normal range
Body temperature
Normal body temperature
Other appropriate diagnostic tests based on history and physical exam
Unremarkable
CBC=complete blood count.
<END OF TEXT ON SCREEN>
<VO: Dr. Nguyen>
Dana’s CBC results were normal. She also did not have a fever and had an unremarkable history and physical exam. Based on her responses and diagnostic assessments, it looks like Dana has Grade 3 diarrhea that is not caused by infection.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Summary of evaluation
Grade 3 diarrhea
No infectious causes
No additional cholinergic symptoms
Abdominal cramping
Diarrhea
Salivation
<END OF TEXT ON SCREEN>
<VO: Dr. Nguyen>
After Dana’s healthcare provider rules out infectious causes, Dana begins taking loperamide as directed by her oncology nurse. Dana’s healthcare team continues to monitor her diarrhea. By day 17, her diarrhea has reduced to Grade 2.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Day 15
- Reports Grade 3 diarrhea
- Infectious causes ruled out
- Begins loperamide use
Day 17
- Decreased to Grade 2 diarrhea
- Continues loperamide use
Cycle 2
Day 1
- Decreased to Grade 1 diarrhea
- Continues loperamide use
<VO: Dr. Nguyen>
On cycle 2, day 1, Dana’s diarrhea has decreased to Grade 1, and she continues to take loperamide.
<TEXT ON-SCREEN>
Cycle 2
Day 8
- Diarrhea has resolved
- Stops loperamide use
<VO: Dr. Nguyen>
On cycle 2, day 8, her diarrhea has resolved, and she ceases loperamide use.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Nguyen>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
PATIENT CASE: CLARA
<VO: Dr. Schultz>
Let’s look at another patient case, this time for an HR+/HER2- metastatic breast cancer patient named Clara.
<END OF TEXT ON SCREEN>
<CAMERA CUTS TO DR. SCHULTZ>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Actor portrayal
Clara
Age: 66
Race/ethnicity: African American
Has fast progressing HR+/HER2- mBC
Metastatic treatment history:
- Endocrine therapy + CDK4/6 inhibitor
- 2 chemotherapies
Hypothetical patient case for illustrative purposes only. Information in this program does not constitute the provision of medical advice and should not substitute for clinical decision-making.
CDK4/6=cyclin-dependent kinases 4/6; HER2-=human epidermal growth factor receptor 2-negative; HR+=hormone receptor-positive; mBC=metastatic breast cancer.
<VO: Dr. Schultz>
Clara is a 66-year-old African American woman with fast progressing HR+/HER2- metastatic breast cancer. She previously underwent combination treatment with an endocrine therapy and CDK4/6 inhibitor followed by 2 chemotherapies. Following her TRODELVY prescription, Clara’s care team has had conversations with her to help her prepare for treatment.
<END OF TEXT ON SCREEN>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Actor portrayal
Clara
- Age: 66
- Race/ethnicity: African American
- Adverse reactions experienced on prior therapies:
- Nausea
- Neutropenia
- Diarrhea
- Peripheral neuropathy
- Alopecia
<VO: Dr. Schultz>
Prior to starting on TRODELVY, Clara is given premedications for the prevention of infusion-related reactions and nausea and vomiting. Her healthcare team notes that with previous treatments, she experienced nausea, neutropenia, diarrhea, peripheral neuropathy, and alopecia. They counsel her on what serious and common adverse reactions can occur with TRODELVY and when to call her healthcare team.
Clara’s doctor plans to administer primary prophylactic G-CSF in her first cycle of TRODELVY since Clara has at least 2 risk factors for febrile neutropenia per the TRODELVY Prescribing Information: she’s an older patient and has experienced previous neutropenia.
<END OF TEXT ON SCREEN>
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Clara’s first cycle of TRODELVY
DOSE 1
DOSE 2
<VO: Dr. Schultz>
On Day 8 of her first cycle with TRODELVY—and before her doctor has administered primary prophylactic G-CSF—Clara's healthcare team monitors her blood cell count and finds that her ANC is 900 per cubic millimeter. This causes Clara’s healthcare team to interrupt her first cycle, and she does not receive her scheduled second dose.
<END OF TEXT ON SCREEN>
<CAMERA CUTS TO BOTH EXPERTS, WIDE SHOT>
<VO: Dr. Schultz>
At this point, there are a few things I would ask Clara, such as:
Has she experienced fatigue, weakness, or trouble breathing?
<TEXT ON-SCREEN>
Fatigue, weakness, or trouble breathing?
<VO: Dr. Schultz>
Any new symptoms, such as cough, diarrhea, vomiting, chills, fever, or burning or pain when you urinate?
<TEXT ON-SCREEN>
Fatigue, weakness, or trouble breathing?
Any new symptoms, such as cough, diarrhea, vomiting, chills, fever, or burning or pain when you urinate?
<VO: Dr. Schultz>
Any wounds that haven’t healed properly?
<TEXT ON-SCREEN>
Fatigue, weakness, or trouble breathing?
Any new symptoms, such as cough, diarrhea, vomiting, chills, fever, or burning or pain when you urinate?
Any wounds that haven’t healed properly?
<VO: Dr. Schultz>
Clara reports still feeling fatigued but otherwise reports no other changes or symptoms.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Fatigue, weakness, or trouble breathing?
Still feeling fatigued
Any new symptoms, such as cough, diarrhea, vomiting, chills, fever, or burning or pain when you urinate?
No
Any wounds that haven’t healed properly?
No
<END OF TEXT ON SCREEN>
<VO: Dr. Schultz>
Diagnostic assessments may also be required such as repeat CBCs, body temperature, or other tests based on her history and physical exam.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Possible diagnostic assessments
Repeat CBC with differential when indicated
ANC on Day 8: 900/mm3
Body temperature
Normal
Other appropriate diagnostic tests based on history and physical exam
Unremarkable
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Schultz>
<CAMERA CUTS TO BOTH EXPERTS, WIDE SHOT>
<VO: Dr. Nguyen>
Based on her responses and diagnostic assessments, it looks like Clara has Grade 3 neutropenia and is afebrile.
<TEXT ANIMATES ON SCREEN>
<TEXT ON-SCREEN>
Summary of evaluation
Grade 3 neutropenia
Decreased neutrophil count
No fever
Fatigue
<VO: Dr. Nguyen>
How did her care team go about addressing Clara’s neutropenia?
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Nguyen>
<GRAPHICS AND TEXT ANIMATE ON SCREEN>
<TEXT ON SCREEN>
Action plan for Clara:
<VO: Dr. Schultz>
Since her ANC is below 1000, TRODELVY is withheld for her day 8 dose and until her ANC is at least 1000/mm3. G-CSF is administered during treatment as clinically indicated. Although Clara is afebrile, her healthcare team continues to monitor her blood cell counts and body temperature.
<TEXT ON SCREEN>
Withhold TRODELVY until ANC ≥1000/mm3 for Day 8 dose
Administer G-CSF during treatment as clinically indicated
Continue to monitor cell counts and body temperature
Clara's neutropenia was graded according to NCI CTCAE v5.0
Does not constitute medical advice and should not substitute for clinical decision-making.
<END OF TEXT ON SCREEN>
<VO: Dr. Schultz>
Clara’s physician chose to give her long-acting G-CSF on Day 8 to help treat her neutropenia.
In my practice, I may consider short-acting G-CSF instead.
<CAMERA CUTS TO BOTH EXPERTS, WIDE SHOT>
<VO: Dr. Schultz>
As discussed earlier, determining the best path forward is a case-by-case decision.
<CAMERA CUTS TO DR. SCHULTZ>
<VO: Dr. Schultz>
Clara’s healthcare team continues to monitor her blood counts. On Day 15, a CBC reveals resolution of neutropenia.
<TEXT ANIMATES ON SCREEN>
<TEXT ON SCREEN>
Day 15
- CBC reveals resolution of neutropenia
- Resumes treatment with TRODELVY at 10 mg/kg
- Receives G-CSF as prophylaxis for subsequent cycles of TRODELVY
<VO: Dr. Schultz>
At this point, Clara’s doctor decides to resume Clara’s treatment with TRODELVY at her original dose of 10 milligrams per kilogram, starting on Day 1 of Cycle 2. For each cycle of TRODELVY going forward, Clara receives long-acting G-CSF on Day 9 as prophylaxis.
<END OF TEXT ON SCREEN>
<CAMERA CUTS TO BOTH EXPERTS, WIDE SHOT>
<VO: Dr. Schultz>
Clara's healthcare team addressed her neutropenia early and were able to implement established strategies to help manage it.
<TEXT ANIMATES ON SCREEN>
<TEXT ON SCREEN>
MONITOR
<END OF TEXT ON SCREEN>
<VO: Dr. Schultz>
Since she did not experience prolonged Grade 3 neutropenia, Clara is an appropriate candidate to continue on therapy at her original dose.
However, it’s still important to continue to monitor Clara’s blood cell counts periodically.
<TEXT ANIMATES ON SCREEN>
<TEXT ON SCREEN>
Monitor blood cell counts periodically during treatment
Instruct and remind patients to contact their healthcare provider immediately if they experience fever, chills, or other signs of infection.
<VO: Dr. Schultz>
She should contact her healthcare provider immediately if she experiences fever, chills, or other signs of infection.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Schultz>
<CAMERA CUTS TO BOTH EXPERTS, WIDE SHOT>
<TEXT ANIMATES ON SCREEN>
<TEXT ON SCREEN>
Every patient case is different, and patient results may vary.
<VO: Dr. Nguyen>
With both Dana and Clara, we can see that early attention from their care teams helped them resolve certain adverse reactions and continue treatment with TRODELVY as clinically appropriate.
<END OF VO: Dr. Nguyen>
<VO: Dr. Schultz>
Keep in mind that every patient case is different, and patient results may vary.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Schultz>
<VO: Dr. Nguyen>
Now that we’ve seen how Clara and Dana did on treatment, are there any final tips you have for cultivating a relationship with your patients?
<END OF VO: Dr. Nguyen>
<VO: Dr. Schultz>
I just want to emphasize again the importance of open and honest communication.
<END OF VO: Dr. Schultz>
<VO: Dr. Nguyen>
I couldn’t agree more. Listening to and answering patients’ questions is so important. It helps me to develop a stronger relationship with them and have better, more proactive conversations.
Setting expectations and developing adverse reaction management plans can help patients feel more confident in their treatment plan. It could also help them potentially continue therapy if clinically appropriate.
<END OF VO: Dr. Nguyen>
<TEXT ANIMATES ON SCREEN>
<TEXT ON SCREEN>
Watch The Difference of Disease: TRODELVY Case Studies
Keep watching for continued Important Safety Information.
<VO: Dr. Schultz>
To learn more about TRODELVY clinical trials, see our video The Difference of Disease: TRODELVY Case Studies.
<END OF VO: Dr. Schultz>
<VO: Dr. Nguyen>
Please keep watching for continued Important Safety Information.
<END OF TEXT ON SCREEN>
<END OF VO: Dr. Nguyen>
<TEXT ON-SCREEN>
IMPORTANT SAFETY INFORMATION (CONT’D)
CONTRAINDICATIONS
- Severe hypersensitivity reaction to TRODELVY.
WARNINGS AND PRECAUTIONS
Neutropenia: Severe, life-threatening, or fatal neutropenia can occur as early as the first cycle of treatment and may require dose modification. Neutropenia occurred in 64% of patients treated with TRODELVY. Grade 3-4 neutropenia occurred in 49% of patients. Febrile neutropenia occurred in 6%. Neutropenic colitis occurred in 1.4%. Primary prophylaxis with G-CSF is recommended starting in the first cycle of treatment in all patients at increased risk of febrile neutropenia, including older patients, patients with previous neutropenia, poor performance status, organ dysfunction, or multiple comorbidities. Monitor absolute neutrophil count (ANC) during treatment. Withhold TRODELVY for ANC below 1500/mm3 on Day 1 of any cycle or neutrophil count below 1000/mm3 on Day 8 of any cycle. Withhold TRODELVY for neutropenic fever. Treat neutropenia with G-CSF and administer prophylaxis in subsequent cycles as clinically indicated or indicated in Table 2 of USPI.
<VO: ISI>
TRODELVY is contraindicated for patients with severe hypersensitivity reaction to TRODELVY.
Warnings and precautions for TRODELVY include neutropenia, diarrhea, hypersensitivity and infusion-related reactions, nausea and vomiting, increased risk of adverse reactions in patients with reduced UGT1A1 activity, and embryo-fetal toxicity.
Neutropenia: Severe, life-threatening, or fatal neutropenia can occur as early as the first cycle of treatment and may require dose modification. Neutropenia occurred in 64% of patients treated with TRODELVY. Grade 3-4 neutropenia occurred in 49% of patients. Febrile neutropenia occurred in 6%. Neutropenic colitis occurred in 1.4%. Primary prophylaxis with G-CSF is recommended starting in the first cycle of treatment in all patients at increased risk of febrile neutropenia, including older patients, patients with previous neutropenia, poor performance status, organ dysfunction, or multiple comorbidities. Monitor absolute neutrophil count (ANC) during treatment. Withhold TRODELVY for ANC below 1500/mm3 on Day 1 of any cycle or below 1000/mm3 on Day 8 of any cycle. Withhold TRODELVY for neutropenic fever. Treat neutropenia with G-CSF and administer prophylaxis in subsequent cycles as clinically indicated or indicated in Table 2 of USPI.
<TEXT ON-SCREEN>
IMPORTANT SAFETY INFORMATION (CONT’D)
WARNINGS AND PRECAUTIONS
Diarrhea: Diarrhea occurred in 64% of all patients treated with TRODELVY. Grade 3-4 diarrhea occurred in 11% of patients. One patient had intestinal perforation following diarrhea. Diarrhea that led to dehydration and subsequent acute kidney injury occurred in 0.7% of all patients. Withhold TRODELVY for Grade 3-4 diarrhea and resume when resolved to ≤Grade 1. At onset, evaluate for infectious causes and if negative, promptly initiate loperamide, 4 mg initially followed by 2 mg with every episode of diarrhea for a maximum of 16 mg daily. Discontinue loperamide 12 hours after diarrhea resolves. Additional supportive measures (e.g., fluid and electrolyte substitution) may also be employed as clinically indicated. Patients who exhibit an excessive cholinergic response to treatment can receive appropriate premedication (e.g., atropine) for subsequent treatments.
<VO: ISI>
Diarrhea: Diarrhea occurred in 64% of all patients treated with TRODELVY. Grade 3-4 diarrhea occurred in 11% of patients. One patient had intestinal perforation following diarrhea. Diarrhea that led to dehydration and subsequent acute kidney injury occurred in 0.7% of all patients. Withhold TRODELVY for Grade 3-4 diarrhea and resume when resolved to ≤Grade 1. At onset, evaluate for infectious causes and if negative, promptly initiate loperamide, 4 mg initially followed by 2 mg with every episode of diarrhea for a maximum of 16 mg daily. Discontinue loperamide 12 hours after diarrhea resolves. Additional supportive measures (for example, fluid and electrolyte substitution) may also be employed as clinically indicated. Patients who exhibit an excessive cholinergic response to treatment can receive appropriate premedication (for example, atropine) for subsequent treatments.
<TEXT ON-SCREEN>
IMPORTANT SAFETY INFORMATION (CONT’D)
WARNINGS AND PRECAUTIONS
Hypersensitivity and Infusion-Related Reactions: TRODELVY can cause serious hypersensitivity reactions including life-threatening anaphylactic reactions. Severe signs and symptoms included cardiac arrest, hypotension, wheezing, angioedema, swelling, pneumonitis, and skin reactions. Hypersensitivity reactions within 24 hours of dosing occurred in 35% of patients. Grade 3-4 hypersensitivity occurred in 2% of patients. The incidence of hypersensitivity reactions leading to permanent discontinuation of TRODELVY was 0.2%. The incidence of anaphylactic reactions was 0.2%. Pre-infusion medication is recommended. Have medications and emergency equipment to treat such reactions available for immediate use. Observe patients closely for hypersensitivity and infusion-related reactions during each infusion and for at least 30 minutes after completion of each infusion. Permanently discontinue TRODELVY for Grade 4 infusion-related reactions.
<VO: ISI>
Hypersensitivity and Infusion-Related Reactions: TRODELVY can cause serious hypersensitivity reactions including life-threatening anaphylactic reactions. Severe signs and symptoms included cardiac arrest, hypotension, wheezing, angioedema, swelling, pneumonitis, and skin reactions. Hypersensitivity reactions within 24 hours of dosing occurred in 35% of patients. Grade 3-4 hypersensitivity occurred in 2% of patients. The incidence of hypersensitivity reactions leading to permanent discontinuation of TRODELVY was 0.2%. The incidence of anaphylactic reactions was 0.2%. Pre-infusion medication is recommended. Have medications and emergency equipment to treat such reactions available for immediate use. Observe patients closely for hypersensitivity and infusion-related reactions during each infusion and for at least 30 minutes after completion of each infusion. Permanently discontinue TRODELVY for Grade 4 infusion-related reactions.
<TEXT ON-SCREEN>
IMPORTANT SAFETY INFORMATION (CONT’D)
WARNINGS AND PRECAUTIONS
Nausea and Vomiting: TRODELVY is emetogenic and can cause severe nausea and vomiting. Nausea occurred in 64% of all patients treated with TRODELVY and Grade 3-4 nausea occurred in 3% of these patients. Vomiting occurred in 35% of patients and Grade 3-4 vomiting occurred in 2% of these patients. Premedicate with a two or three drug combination regimen (e.g., dexamethasone with either a 5-HT3 receptor antagonist or an NK1 receptor antagonist as well as other drugs as indicated) for prevention of chemotherapy-induced nausea and vomiting (CINV). Withhold TRODELVY doses for Grade 3 nausea or Grade 3-4 vomiting and resume with additional supportive measures when resolved to less than or equal to Grade 1. Additional antiemetics and other supportive measures may also be employed as clinically indicated. All patients should be given take-home medications with clear instructions for prevention and treatment of nausea and vomiting.
<VO: ISI>
Nausea and Vomiting: TRODELVY is emetogenic and can cause severe nausea and vomiting. Nausea occurred in 64% of all patients treated with TRODELVY and Grade 3-4 nausea occurred in 3% of these patients. Vomiting occurred in 35% of patients and Grade 3-4 vomiting occurred in 2% of these patients. Premedicate with a two or three drug combination regimen (for example, dexamethasone with either a 5-HT3 receptor antagonist or an NK1 receptor antagonist as well as other drugs as indicated) for prevention of chemotherapy-induced nausea and vomiting (CINV). Withhold TRODELVY doses for Grade 3 nausea or Grade 3-4 vomiting and resume with additional supportive measures when resolved to Grade ≤1. Additional antiemetics and other supportive measures may also be employed as clinically indicated. All patients should be given take-home medications with clear instructions for prevention and treatment of nausea and vomiting.
<TEXT ON-SCREEN>
IMPORTANT SAFETY INFORMATION (CONT’D)
WARNINGS AND PRECAUTIONS
Increased Risk of Adverse Reactions in Patients with Reduced UGT1A1 Activity: Patients homozygous for the uridine diphosphate-glucuronosyl transferase 1A1 (UGT1A1)*28 allele are at increased risk for neutropenia, febrile neutropenia, and anemia and may be at increased risk for other adverse reactions with TRODELVY. The incidence of Grade 3-4 neutropenia was 58% in patients homozygous for the UGT1A1*28, 49% in patients heterozygous for the UGT1A1*28 allele, and 43% in patients homozygous for the wild-type allele. The incidence of Grade 3-4 anemia was 21% in patients homozygous for the UGT1A1*28 allele, 10% in patients heterozygous for the UGT1A1*28 allele, and 9% in patients homozygous for the wild-type allele. Closely monitor patients with known reduced UGT1A1 activity for adverse reactions. Withhold or permanently discontinue TRODELVY based on clinical assessment of the onset, duration and severity of the observed adverse reactions in patients with evidence of acute early-onset or unusually severe adverse reactions, which may indicate reduced UGT1A1 function.
<VO: ISI>
Increased Risk of Adverse Reactions in Patients with Reduced UGT1A1 Activity: Patients homozygous for the uridine diphosphate-glucuronosyl transferase 1A1 or UGT1A1*28 allele are at increased risk for neutropenia, febrile neutropenia, and anemia and may be at increased risk for other adverse reactions with TRODELVY. The incidence of Grade 3-4 neutropenia was 58% in patients homozygous for the UGT1A1*28, 49% in patients heterozygous for the UGT1A1*28 allele, and 43% in patients homozygous for the wild-type allele. The incidence of Grade 3-4 anemia was 21% in patients homozygous for the UGT1A1*28 allele, 10% in patients heterozygous for the UGT1A1*28 allele, and 9% in patients homozygous for the wild-type allele. Closely monitor patients with known reduced UGT1A1 activity for adverse reactions. Withhold or permanently discontinue TRODELVY based on clinical assessment of the onset, duration and severity of the observed adverse reactions in patients with evidence of acute early-onset or unusually severe adverse reactions, which may indicate reduced UGT1A1 function.
<TEXT ON-SCREEN>
IMPORTANT SAFETY INFORMATION (CONT’D)
WARNINGS AND PRECAUTIONS
Embryo-Fetal Toxicity: Based on its mechanism of action, TRODELVY can cause teratogenicity and/or embryo-fetal lethality when administered to a pregnant woman. TRODELVY contains a genotoxic component, SN-38, and targets rapidly dividing cells. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TRODELVY and for 6 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with TRODELVY and for 3 months after the last dose.
<VO: ISI>
Embryo-Fetal Toxicity: Based on its mechanism of action, TRODELVY can cause teratogenicity and/or embryo-fetal lethality when administered to a pregnant woman. TRODELVY contains a genotoxic component, SN-38, and targets rapidly dividing cells. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TRODELVY and for 6 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with TRODELVY and for 3 months after the last dose.
<TEXT ON-SCREEN>
IMPORTANT SAFETY INFORMATION (CONT’D)
ADVERSE REACTIONS
In the pooled safety population, the most common (≥25%) adverse reactions including laboratory abnormalities were decreased leukocyte count (84%), decreased neutrophil count (75%), decreased hemoglobin (69%), diarrhea (64%), nausea (64%), decreased lymphocyte count (63%), fatigue (51%), alopecia (45%), constipation (37%), increased glucose (37%), decreased albumin (35%), vomiting (35%), decreased appetite (30%), decreased creatinine clearance (28%), increased alkaline phosphatase (28%), decreased magnesium (27%), decreased potassium (26%), and decreased sodium (26%).
<VO: ISI>
The following adverse reactions were observed with TRODELVY.
In the pooled safety population, the most common (≥25%) adverse reactions including laboratory abnormalities were decreased leukocyte count (84%), decreased neutrophil count (75%), decreased hemoglobin (69%), diarrhea (64%), nausea (64%), decreased lymphocyte count (63%), fatigue (51%), alopecia (45%), constipation (37%), increased glucose (37%), decreased albumin (35%), vomiting (35%), decreased appetite (30%), decreased creatinine clearance (28%), increased alkaline phosphatase (28%), decreased magnesium (27%), decreased potassium (26%), and decreased sodium (26%).
<TEXT ON-SCREEN>
IMPORTANT SAFETY INFORMATION (CONT’D)
ADVERSE REACTIONS
In the ASCENT study (locally advanced or metastatic triple-negative breast cancer), the most common adverse reactions (incidence ≥25%) were fatigue, diarrhea, nausea, alopecia, constipation, vomiting, abdominal pain, and decreased appetite. The most frequent serious adverse reactions (SAR) incidence (>1%) were neutropenia (7%), diarrhea (4%), and pneumonia (3%). SAR were reported in 27% of patients, and 5% discontinued therapy due to adverse reactions. The most common Grade 3-4 lab abnormalities (incidence ≥25%) in the ASCENT study were reduced neutrophils, leukocytes, and lymphocytes.
In the TROPiCS-02 study (locally advanced or metastatic HR-positive, HER2-negative breast cancer), the most common adverse reactions (incidence ≥25%) were diarrhea, fatigue, nausea, alopecia, and constipation. The most frequent serious adverse reactions (SAR) (>1%) were diarrhea (5%), febrile neutropenia (4%), neutropenia (3%), abdominal pain, colitis, neutropenic colitis, pneumonia, and vomiting (each 2%). SAR were reported in 28% of patients, and 6% discontinued therapy due to adverse reactions. The most common Grade 3-4 lab abnormalities (incidence ≥25%) in the TROPiCS-02 study were reduced neutrophils and leukocytes.
<VO: ISI>
In the ASCENT study (locally advanced or metastatic triple-negative breast cancer), the most common adverse reactions (incidence ≥25%) were fatigue, diarrhea, nausea, alopecia, constipation, vomiting, abdominal pain, and decreased appetite. The most frequent serious adverse reactions (incidence (>1%) were neutropenia (7%), diarrhea (4%), and pneumonia (3%). Serious adverse reactions were reported in 27% of patients, and 5% of patients discontinued therapy due to adverse reactions. The most common Grade 3-4 lab abnormalities (incidence ≥25%) in the ASCENT study were reduced neutrophils, leukocytes, and lymphocytes.
In the TROPiCS-02 study (locally advanced or metastatic HR-positive, HER2-negative breast cancer), the most common adverse reactions (incidence ≥25%) were diarrhea, fatigue, nausea, alopecia, and constipation. The most frequent serious adverse reactions (SAR) (>1%) were diarrhea (5%), febrile neutropenia (4%), neutropenia (3%), abdominal pain, colitis, neutropenic colitis, pneumonia, and vomiting (each 2%). SAR were reported in 28% of patients, and 6% discontinued therapy due to adverse reactions. The most common Grade 3-4 lab abnormalities (incidence ≥25%) in the TROPiCS-02 study were reduced neutrophils and leukocytes.
<TEXT ON-SCREEN>
IMPORTANT SAFETY INFORMATION (CONT’D)
DRUG INTERACTIONS
UGT1A1 Inhibitors: Concomitant administration of TRODELVY with inhibitors of UGT1A1 may increase the incidence of adverse reactions due to potential increase in systemic exposure to SN-38. Avoid administering UGT1A1 inhibitors with TRODELVY.
UGT1A1 Inducers: Exposure to SN-38 may be reduced in patients concomitantly receiving UGT1A1 enzyme inducers. Avoid administering UGT1A1 inducers with TRODELVY.
Please see link provided for full Prescribing Information, including BOXED WARNING, also available at TRODELVYhcp.com
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
<VO: ISI>
Drug interactions for TRODELVY include UGT1A1 inhibitors and UGT1A1 inducers.
UGT1A1 Inhibitors: Concomitant administration of TRODELVY with inhibitors of UGT1A1 may increase the incidence of adverse reactions due to potential increase in systemic exposure to SN-38. Avoid administering UGT1A1 inhibitors with TRODELVY.
UGT1A1 Inducers: Exposure to SN-38 may be reduced in patients concomitantly receiving UGT1A1 enzyme inducers. Avoid administering UGT1A1 inducers with TRODELVY.
Please see full Prescribing Information, including BOXED WARNING.
<END OF TEXT ON SCREEN>
<TEXT ON SCREEN>
This information does not constitute the provision of medical advice and should not substitute for clinical decision-making.
TRODELVY, the TRODELVY logo, GILEAD, and the GILEAD logo are trademarks of Gilead Sciences, Inc., or its related companies. All other marks are the property of their respective owners.
© 2025 Gilead Sciences, Inc. All rights reserved. US-TROP-1903 12/25
[IMAGE: TRODELVY LOGO]
[IMAGE: GILEAD LOGO]
<END OF TEXT ON SCREEN>
Schedule a visit with your Gilead Oncology Nurse Educator (ONE) or sign up to receive more information from your ONE
ANC=absolute neutrophil count; ASCO=American Society of Clinical Oncology; BICR=blinded independent central review; BRCA=breast cancer gene; CAP=College of American Pathologists; CD4=cluster of differentiation 4; CDK4/6i=cyclin-dependent kinase 4/6 inhibitor; DOR=duration of response; ECOG=Eastern Cooperative Oncology Group; FN=febrile neutropenia; G-CSF=granulocyte colony-stimulating factor; HER2=human epidermal growth factor receptor 2; HR=hormone receptor; IHC=immunohistochemistry; IV=intravenous; mBC=metastatic breast cancer; mTNBC=metastatic triple-negative breast cancer; ORR=objective response rate; OS=overall survival; PFS=progression-free survival; PRO=patient-reported outcome; RECIST=Response Evaluation Criteria in Solid Tumors.
References: 1. TRODELVY. Prescribing Information. Gilead Sciences, Inc.; March 2025. 2. Rugo HS, Bardia A, Marmé F, et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;402(10411):1423-1433. 3. Rugo HS, Tolaney SM, Loirat D, et al. Safety analyses from the phase 3 ASCENT trial of sacituzumab govitecan in metastatic triple-negative breast cancer. NPJ Breast Cancer. 2022;8(1):98. doi:10.1038/s41523-022-00467-1 4. Data on file. Gilead Sciences, Inc.; March 2025. 5. National Cancer Institute, Division of Cancer Treatment & Diagnosis (DCTD). Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. National Institutes of Health. Published November 27, 2017. Accessed October 10, 2025.
Continue exploring adverse reaction management strategies
Select another topic
Scroll left to review
TRODELVY® (sacituzumab govitecan-hziy) is a Trop-2-directed antibody and topoisomerase inhibitor conjugate indicated for the treatment of adult patients with:
- Unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
- Unresectable locally advanced or metastatic hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative (IHC 0, IHC 1+ or IHC 2+/ISH–) breast cancer who have received endocrine-based therapy and at least two additional systemic therapies in the metastatic setting.
Important Safety Information
Tap for Important Safety Information, including BOXED WARNING: Neutropenia and Diarrhea.
Boxed Warning: neutropenia and diarrhea
- TRODELVY can cause severe, life-threatening, or fatal neutropenia. Withhold TRODELVY for absolute neutrophil count below 1500/mm3 or neutropenic fever. Monitor blood cell counts periodically during treatment. Primary prophylaxis with G-CSF is recommended for all patients at increased risk of febrile neutropenia. Initiate anti-infective treatment in patients with febrile neutropenia without delay.
- TRODELVY can cause severe diarrhea. Monitor patients with diarrhea and give fluid and electrolytes as needed. At the onset of diarrhea, evaluate for infectious causes and, if negative, promptly initiate loperamide. If severe diarrhea occurs, withhold TRODELVY until resolved to ≤Grade 1 and reduce subsequent doses.
Contraindications
- Severe hypersensitivity reaction to TRODELVY.
Warnings and precautions
Neutropenia: Severe, life-threatening, or fatal neutropenia can occur as early as the first cycle of treatment and may require dose modification. Neutropenia occurred in 64% of patients treated with TRODELVY. Grade 3-4 neutropenia occurred in 49% of patients. Febrile neutropenia occurred in 6%. Neutropenic colitis occurred in 1.4%. Primary prophylaxis with G-CSF is recommended starting in the first cycle of treatment in all patients at increased risk of febrile neutropenia, including older patients, patients with previous neutropenia, poor performance status, organ dysfunction, or multiple comorbidities. Monitor absolute neutrophil count (ANC) during treatment. Withhold TRODELVY for ANC below 1500/mm3 on Day 1 of any cycle or below 1000/mm3 on Day 8 of any cycle. Withhold TRODELVY for neutropenic fever. Treat neutropenia with G-CSF and administer prophylaxis in subsequent cycles as clinically indicated or indicated in Table 2 of USPI.
Diarrhea: Diarrhea occurred in 64% of all patients treated with TRODELVY. Grade 3-4 diarrhea occurred in 11% of patients. One patient had intestinal perforation following diarrhea. Diarrhea that led to dehydration and subsequent acute kidney injury occurred in 0.7% of all patients. Withhold TRODELVY for Grade 3-4 diarrhea and resume when resolved to ≤Grade 1. At onset, evaluate for infectious causes and if negative, promptly initiate loperamide, 4 mg initially followed by 2 mg with every episode of diarrhea for a maximum of 16 mg daily. Discontinue loperamide 12 hours after diarrhea resolves. Additional supportive measures (e.g., fluid and electrolyte substitution) may also be employed as clinically indicated. Patients who exhibit an excessive cholinergic response to treatment can receive appropriate premedication (e.g., atropine) for subsequent treatments.
Hypersensitivity and Infusion-Related Reactions: TRODELVY can cause serious hypersensitivity reactions including life-threatening anaphylactic reactions. Severe signs and symptoms included cardiac arrest, hypotension, wheezing, angioedema, swelling, pneumonitis, and skin reactions. Hypersensitivity reactions within 24 hours of dosing occurred in 35% of patients. Grade 3-4 hypersensitivity occurred in 2% of patients. The incidence of hypersensitivity reactions leading to permanent discontinuation of TRODELVY was 0.2%. The incidence of anaphylactic reactions was 0.2%. Pre-infusion medication is recommended. Have medications and emergency equipment to treat such reactions available for immediate use. Observe patients closely for hypersensitivity and infusion-related reactions during each infusion and for at least 30 minutes after completion of each infusion. Permanently discontinue TRODELVY for Grade 4 infusion-related reactions.
Nausea and Vomiting: TRODELVY is emetogenic and can cause severe nausea and vomiting. Nausea occurred in 64% of all patients treated with TRODELVY and Grade 3-4 nausea occurred in 3% of these patients. Vomiting occurred in 35% of patients and Grade 3-4 vomiting occurred in 2% of these patients. Premedicate with a two or three drug combination regimen (e.g., dexamethasone with either a 5-HT3 receptor antagonist or an NK1 receptor antagonist as well as other drugs as indicated) for prevention of chemotherapy-induced nausea and vomiting (CINV). Withhold TRODELVY doses for Grade 3 nausea or Grade 3-4 vomiting and resume with additional supportive measures when resolved to Grade ≤1. Additional antiemetics and other supportive measures may also be employed as clinically indicated. All patients should be given take-home medications with clear instructions for prevention and treatment of nausea and vomiting.
Increased Risk of Adverse Reactions in Patients with Reduced UGT1A1 Activity: Patients homozygous for the uridine diphosphate-glucuronosyl transferase 1A1 (UGT1A1)*28 allele are at increased risk for neutropenia, febrile neutropenia, and anemia and may be at increased risk for other adverse reactions with TRODELVY. The incidence of Grade 3-4 neutropenia was 58% in patients homozygous for the UGT1A1*28, 49% in patients heterozygous for the UGT1A1*28 allele, and 43% in patients homozygous for the wild-type allele. The incidence of Grade 3-4 anemia was 21% in patients homozygous for the UGT1A1*28 allele, 10% in patients heterozygous for the UGT1A1*28 allele, and 9% in patients homozygous for the wild-type allele. Closely monitor patients with known reduced UGT1A1 activity for adverse reactions. Withhold or permanently discontinue TRODELVY based on clinical assessment of the onset, duration and severity of the observed adverse reactions in patients with evidence of acute early-onset or unusually severe adverse reactions, which may indicate reduced UGT1A1 function.
Embryo-Fetal Toxicity: Based on its mechanism of action, TRODELVY can cause teratogenicity and/or embryo-fetal lethality when administered to a pregnant woman. TRODELVY contains a genotoxic component, SN-38, and targets rapidly dividing cells. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TRODELVY and for 6 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with TRODELVY and for 3 months after the last dose.
Adverse Reactions
In the pooled safety population, the most common (≥25%) adverse reactions including laboratory abnormalities were decreased leukocyte count (84%), decreased neutrophil count (75%), decreased hemoglobin (69%), diarrhea (64%), nausea (64%), decreased lymphocyte count (63%), fatigue (51%), alopecia (45%), constipation (37%), increased glucose (37%), decreased albumin (35%), vomiting (35%), decreased appetite (30%), decreased creatinine clearance (28%), increased alkaline phosphatase (28%), decreased magnesium (27%), decreased potassium (26%), and decreased sodium (26%).
In the ASCENT study (locally advanced or metastatic triple-negative breast cancer), the most common adverse reactions (incidence ≥25%) were fatigue, diarrhea, nausea, alopecia, constipation, vomiting, abdominal pain, and decreased appetite. The most frequent serious adverse reactions (SAR) (>1%) were neutropenia (7%), diarrhea (4%), and pneumonia (3%). SAR were reported in 27% of patients, and 5% discontinued therapy due to adverse reactions. The most common Grade 3-4 lab abnormalities (incidence ≥25%) in the ASCENT study were reduced neutrophils, leukocytes, and lymphocytes.
In the TROPiCS-02 study (locally advanced or metastatic HR-positive, HER2-negative breast cancer), the most common adverse reactions (incidence ≥25%) were diarrhea, fatigue, nausea, alopecia, and constipation. The most frequent serious adverse reactions (SAR) (>1%) were diarrhea (5%), febrile neutropenia (4%), neutropenia (3%), abdominal pain, colitis, neutropenic colitis, pneumonia, and vomiting (each 2%). SAR were reported in 28% of patients, and 6% discontinued therapy due to adverse reactions. The most common Grade 3-4 lab abnormalities (incidence ≥25%) in the TROPiCS-02 study were reduced neutrophils and leukocytes.
Drug Interactions
UGT1A1 Inhibitors: Concomitant administration of TRODELVY with inhibitors of UGT1A1 may increase the incidence of adverse reactions due to potential increase in systemic exposure to SN-38. Avoid administering UGT1A1 inhibitors with TRODELVY.
UGT1A1 Inducers: Exposure to SN-38 may be reduced in patients concomitantly receiving UGT1A1 enzyme inducers. Avoid administering UGT1A1 inducers with TRODELVY.
Please see full Prescribing Information, including BOXED WARNING.





