Scroll left to select an efficacy measure
mOS in pretreated mTNBC (HR-/HER2-)
The only Trop-2–directed ADC with statistically significant OS compared to single-agent chemotherapy1
Median Overall Survival
Scroll left to select an efficacy measure
Median Overall Survival
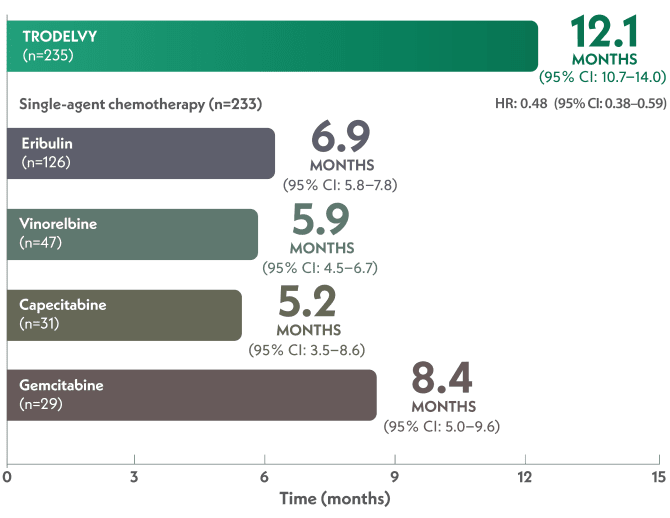
HR: 0.48 (95% CI: 0.38–0.59); P<0.001
mOS in the full population of ASCENT was 11.8 months with TRODELVY (95% CI: 10.5–13.8) (n=267) vs 6.9 months with chemotherapy (95% CI: 5.9–7.6) (n=262); HR: 0.51 (95% CI: 0.41–0.62); P<0.00011
HR: 0.51 (95% CI: 0.41–0.62); P<0.0001
POST HOC SUBGROUP ANALYSIS
Limitation: These results are from a post hoc subgroup analysis of the Phase 3 ASCENT study. The single-agent chemotherapy arms were not powered for statistical analysis or designed to compare against individual agents, and findings should be considered descriptive only. Therefore, the results require cautious interpretation and could represent chance findings.4
88% of patients in the study were brain-met–negative.1,2
Within the single-agent chemotherapy arm, eribulin was the most commonly chosen chemotherapy (n=126), followed by vinorelbine (n=47), capecitabine (n=31), and gemcitabine (n=29).4
OS Across IHC Status
Limitation: These results are from a post hoc subgroup analysis of the Phase 3 ASCENT study, were not powered for statistical analysis, and should be considered descriptive only. The lack of central assessment for HER2 expression and the 21% [n/N=113/529] of patients in the ASCENT full population with missing specific HER2 IHC results are known limitations of this study. Therefore, these results require cautious interpretation and could represent chance findings.5
a79% of patients (n=416) in the ASCENT full population (N=529) were HER2-evaluable by IHC.
Scroll left to review |
mOS in patients treated
|
mOS in patients treated
|
|---|---|---|
HER2 IHC 0(n=293) |
11.3months |
5.9months |
| HR: 0.51 (95% CI: 0.39–0.66) | ||
bHER2-negative status was based on local assessment of the most recent biopsy/pathology report.5
Scroll left to review |
mOS in patients treated
|
mOS in patients treated
|
|---|---|---|
HER2-low (IHC 1+, IHC 2+/ISH-)
|
14.0months |
8.7months |
| HR: 0.43 (95% CI: 0.28-0.67) | ||
bHER2-negative status was based on local assessment of the most recent biopsy/pathology report.5
55 (21%) for TRODELVY vs 58 (22%) for single-agent chemotherapy
HER2-evaluable
by IHC
not evaluable
by IHC
2L=second-line; brain-met=brain metastases; CI=confidence interval; HER2=human epidermal growth factor receptor 2; HR=hazard ratio; IHC=immunohistochemistry; ISH=in situ hybridization; ITT=intent-to-treat; mOS=median overall survival; mTNBC=metastatic triple-negative breast cancer; OS=overall survival; SEER=Surveillance, Epidemiology, and End Results.
References: 1. TRODELVY. Prescribing Information. Gilead Sciences, Inc.; March 2025. 2. Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529-1541. 3. Immunomedics, Inc. An international multi-center, open-label, randomized, phase III trial of sacituzumab govitecan versus treatment of physician choice in patients with metastatic triple-negative cancer who received at least two prior treatments. Published November 18, 2015. Updated June 22, 2017. Accessed May 22, 2025. https://www.nejm.org/doi/suppl/10.1056/NEJMoa2028485/suppl_file/nejmoa2028485_ protocol.pdf 4. O’Shaughnessy J, Punie K, Oliveira M, et al. Assessment of sacituzumab govitecan vs treatment of physician’s choice cohort by agent in the phase 3 ASCENT study of patients with metastatic triple-negative breast cancer. Poster presented at: American Society of Clinical Oncology Annual Meeting; June 4-8, 2021. Poster 1077. https://meetings.asco.org/meetings/2021-asco-annual-meeting/273/program-guide/scheduled-sessions 5. Hurvitz SA, Bardia A, Punie K, et al. Sacituzumab govitecan efficacy in patients with metastatic triple-negative breast cancer by HER2 immunohistochemistry status: findings from the phase 3 ASCENT study. Poster presented at: European Society for Medical Oncology Breast Cancer Congress; May 3-5, 2022; Berlin, Germany. Poster 168P.
Select another topic
Scroll left to select an efficacy measure
TRODELVY® (sacituzumab govitecan-hziy) is a Trop-2-directed antibody and topoisomerase inhibitor conjugate indicated for the treatment of adult patients with:
Tap for Important Safety Information, including BOXED WARNING: Neutropenia and Diarrhea.
Neutropenia: Severe, life-threatening, or fatal neutropenia can occur as early as the first cycle of treatment and may require dose modification. Neutropenia occurred in 64% of patients treated with TRODELVY. Grade 3-4 neutropenia occurred in 49% of patients. Febrile neutropenia occurred in 6%. Neutropenic colitis occurred in 1.4%. Primary prophylaxis with G-CSF is recommended starting in the first cycle of treatment in all patients at increased risk of febrile neutropenia, including older patients, patients with previous neutropenia, poor performance status, organ dysfunction, or multiple comorbidities. Monitor absolute neutrophil count (ANC) during treatment. Withhold TRODELVY for ANC below 1500/mm3 on Day 1 of any cycle or below 1000/mm3 on Day 8 of any cycle. Withhold TRODELVY for neutropenic fever. Treat neutropenia with G-CSF and administer prophylaxis in subsequent cycles as clinically indicated or indicated in Table 2 of USPI.
Diarrhea: Diarrhea occurred in 64% of all patients treated with TRODELVY. Grade 3-4 diarrhea occurred in 11% of patients. One patient had intestinal perforation following diarrhea. Diarrhea that led to dehydration and subsequent acute kidney injury occurred in 0.7% of all patients. Withhold TRODELVY for Grade 3-4 diarrhea and resume when resolved to ≤Grade 1. At onset, evaluate for infectious causes and if negative, promptly initiate loperamide, 4 mg initially followed by 2 mg with every episode of diarrhea for a maximum of 16 mg daily. Discontinue loperamide 12 hours after diarrhea resolves. Additional supportive measures (e.g., fluid and electrolyte substitution) may also be employed as clinically indicated. Patients who exhibit an excessive cholinergic response to treatment can receive appropriate premedication (e.g., atropine) for subsequent treatments.
Hypersensitivity and Infusion-Related Reactions: TRODELVY can cause serious hypersensitivity reactions including life-threatening anaphylactic reactions. Severe signs and symptoms included cardiac arrest, hypotension, wheezing, angioedema, swelling, pneumonitis, and skin reactions. Hypersensitivity reactions within 24 hours of dosing occurred in 35% of patients. Grade 3-4 hypersensitivity occurred in 2% of patients. The incidence of hypersensitivity reactions leading to permanent discontinuation of TRODELVY was 0.2%. The incidence of anaphylactic reactions was 0.2%. Pre-infusion medication is recommended. Have medications and emergency equipment to treat such reactions available for immediate use. Observe patients closely for hypersensitivity and infusion-related reactions during each infusion and for at least 30 minutes after completion of each infusion. Permanently discontinue TRODELVY for Grade 4 infusion-related reactions.
Nausea and Vomiting: TRODELVY is emetogenic and can cause severe nausea and vomiting. Nausea occurred in 64% of all patients treated with TRODELVY and Grade 3-4 nausea occurred in 3% of these patients. Vomiting occurred in 35% of patients and Grade 3-4 vomiting occurred in 2% of these patients. Premedicate with a two or three drug combination regimen (e.g., dexamethasone with either a 5-HT3 receptor antagonist or an NK1 receptor antagonist as well as other drugs as indicated) for prevention of chemotherapy-induced nausea and vomiting (CINV). Withhold TRODELVY doses for Grade 3 nausea or Grade 3-4 vomiting and resume with additional supportive measures when resolved to Grade ≤1. Additional antiemetics and other supportive measures may also be employed as clinically indicated. All patients should be given take-home medications with clear instructions for prevention and treatment of nausea and vomiting.
Increased Risk of Adverse Reactions in Patients with Reduced UGT1A1 Activity: Patients homozygous for the uridine diphosphate-glucuronosyl transferase 1A1 (UGT1A1)*28 allele are at increased risk for neutropenia, febrile neutropenia, and anemia and may be at increased risk for other adverse reactions with TRODELVY. The incidence of Grade 3-4 neutropenia was 58% in patients homozygous for the UGT1A1*28, 49% in patients heterozygous for the UGT1A1*28 allele, and 43% in patients homozygous for the wild-type allele. The incidence of Grade 3-4 anemia was 21% in patients homozygous for the UGT1A1*28 allele, 10% in patients heterozygous for the UGT1A1*28 allele, and 9% in patients homozygous for the wild-type allele. Closely monitor patients with known reduced UGT1A1 activity for adverse reactions. Withhold or permanently discontinue TRODELVY based on clinical assessment of the onset, duration and severity of the observed adverse reactions in patients with evidence of acute early-onset or unusually severe adverse reactions, which may indicate reduced UGT1A1 function.
Embryo-Fetal Toxicity: Based on its mechanism of action, TRODELVY can cause teratogenicity and/or embryo-fetal lethality when administered to a pregnant woman. TRODELVY contains a genotoxic component, SN-38, and targets rapidly dividing cells. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TRODELVY and for 6 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with TRODELVY and for 3 months after the last dose.
In the pooled safety population, the most common (≥25%) adverse reactions including laboratory abnormalities were decreased leukocyte count (84%), decreased neutrophil count (75%), decreased hemoglobin (69%), diarrhea (64%), nausea (64%), decreased lymphocyte count (63%), fatigue (51%), alopecia (45%), constipation (37%), increased glucose (37%), decreased albumin (35%), vomiting (35%), decreased appetite (30%), decreased creatinine clearance (28%), increased alkaline phosphatase (28%), decreased magnesium (27%), decreased potassium (26%), and decreased sodium (26%).
In the ASCENT study (locally advanced or metastatic triple-negative breast cancer), the most common adverse reactions (incidence ≥25%) were fatigue, diarrhea, nausea, alopecia, constipation, vomiting, abdominal pain, and decreased appetite. The most frequent serious adverse reactions (SAR) (>1%) were neutropenia (7%), diarrhea (4%), and pneumonia (3%). SAR were reported in 27% of patients, and 5% discontinued therapy due to adverse reactions. The most common Grade 3-4 lab abnormalities (incidence ≥25%) in the ASCENT study were reduced neutrophils, leukocytes, and lymphocytes.
In the TROPiCS-02 study (locally advanced or metastatic HR-positive, HER2-negative breast cancer), the most common adverse reactions (incidence ≥25%) were diarrhea, fatigue, nausea, alopecia, and constipation. The most frequent serious adverse reactions (SAR) (>1%) were diarrhea (5%), febrile neutropenia (4%), neutropenia (3%), abdominal pain, colitis, neutropenic colitis, pneumonia, and vomiting (each 2%). SAR were reported in 28% of patients, and 6% discontinued therapy due to adverse reactions. The most common Grade 3-4 lab abnormalities (incidence ≥25%) in the TROPiCS-02 study were reduced neutrophils and leukocytes.
UGT1A1 Inhibitors: Concomitant administration of TRODELVY with inhibitors of UGT1A1 may increase the incidence of adverse reactions due to potential increase in systemic exposure to SN-38. Avoid administering UGT1A1 inhibitors with TRODELVY.
UGT1A1 Inducers: Exposure to SN-38 may be reduced in patients concomitantly receiving UGT1A1 enzyme inducers. Avoid administering UGT1A1 inducers with TRODELVY.
Please see full Prescribing Information, including BOXED WARNING.